By Ryan Maher
John B. Goodenough (ΦBK, Yale) was among the three scientists awarded the 2019 Nobel Prize in Chemistry for his role in developing the lithium-ion battery. The powerful, lightweight, and rechargeable battery sparked a revolution in portable electronics and renewable energy, enabling the personal devices ubiquitous to modern living, the storage of solar and wind energy, and the increasing practicality of electric vehicles. He shares the award with M. Stanley Whittingham of the United Kingdom and Akira Yoshino of Japan.
Speaking to the impact of the battery, Olof Ramström of the Nobel Committee said, “It’s all about charging the world and having access to energy wherever we go.”
Goodenough was born in Germany in 1922, making him the oldest recipient of the Nobel Prize at age 97. He served as a meteorologist in the US Army Air Corps during WWII and eventually received his doctorate in physics at the University of Chicago. He subsequently worked at the MIT Lincoln Laboratory, developing the first random-access memory (RAM) for the US Air Force’s SAGE computer, before taking a professorship at Oxford University in 1976.
Goodenough made his pivotal contribution to the lithium-ion battery at Oxford, building off of Whittingham’s work. Whittingham had developed the lithium battery while working for Exxon in the mid 1970s, seeking alternative energy sources in response to the oil embargo. Using metallic lithium as the anode (–) and titanium disulfide as the cathode (+), he created a rechargeable battery that used intercalation (the process of ions attaching to spaces in solid materials) to move lithium ions between either end. Goodenough, then head of Oxford’s Inorganic Chemistry Lab, optimized Whittingham’s battery. He replaced the cathode with cobalt oxide, as a metal oxide would increase the battery’s potential (the difference in charge between its anode and cathode). Goodenough’s battery sported a powerful 4 Volts, and its relatively light weight and high capacity brought it to the cusp of commercial viability.
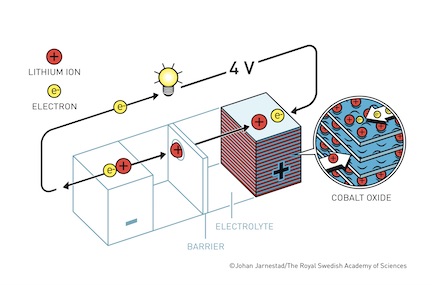
It would take the work of Yoshino to perfect the battery. Building from Goodenough’s innovation, Yoshino replaced the anode with petroleum coke. By removing the metallic lithium, the battery became far safer and longer lasting. Altogether, these three scientists produced a battery that does not degrade as a result of chemical reactions in the anode and cathode, thereby making it functionally viable for far longer periods of time than previous batteries made of lead acid or nickel-cadmium.
Critically, the lithium-ion battery also lends itself well to harnessing renewable energy. As Ramström stated at Goodenough’s Nobel lecture, “We can now increasingly access electricity without access to the grid, wherever we are and whenever we need it. This has indeed changed our lives dramatically.”
Today, Goodenough remains active in his search for a better battery. He has been a professor of mechanical and electrical engineering at the University of Texas at Austin’s Cockrell School of Engineering since 1986. In 2017, he and senior research fellow Maria Helena Braga demonstrated their prototypical glass battery to be a “safe, low-cost, lithium or sodium rechargeable battery of high energy density and long cycle life.” This all-solid-state battery eliminates the dangers associated with liquid electrolytes and offers three times the energy density of today’s batteries, paving the way for the expansion of electric vehicles to the mainstream.
At his press conference in October, Goodenough said of his motivation, “It’s interesting. You work with nice people,” adding in jest, “They do all the hard work, and you sit back and try to take as much credit as you can!”




